
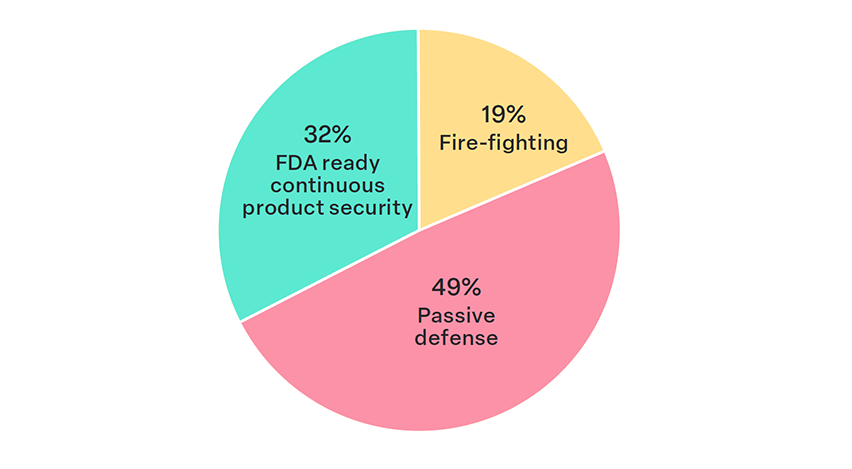
68% of respondents believe their organizations lack a mature product security program, indicating significant room for growth
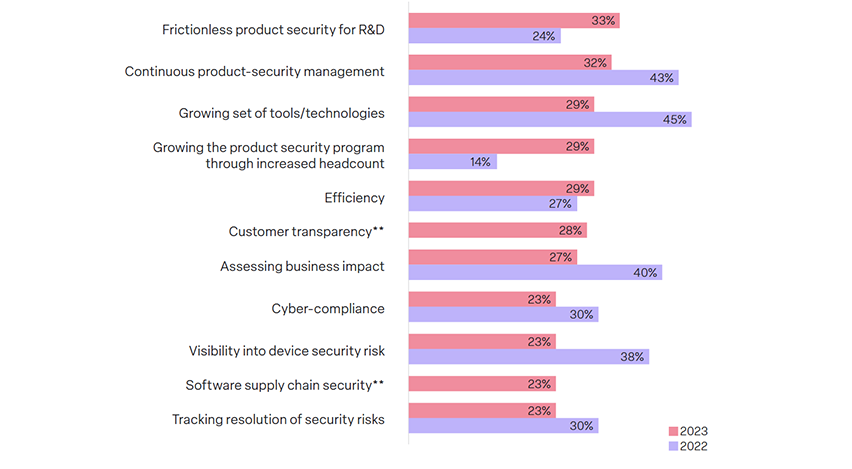
33% of respondents say that their top challenge revolves around streamlining product security activities with R&D teams.
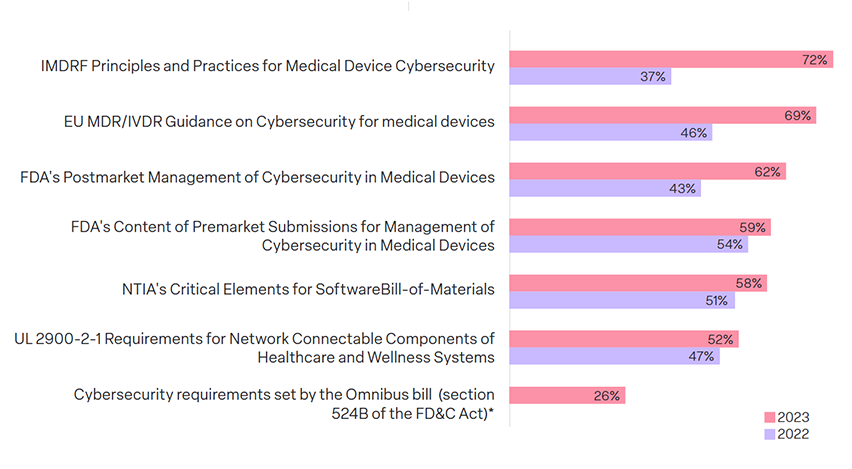
59% are compliant with FDA cybersecurity guidelines– a weak jump from 54% in 2022, even with RTA being spoken about since March.
In this report you’ll learn
-
The state of today’s medical device security
-
Top common security challenges
-
How companies are leveraging product security and compliance